thumbnail
Investments
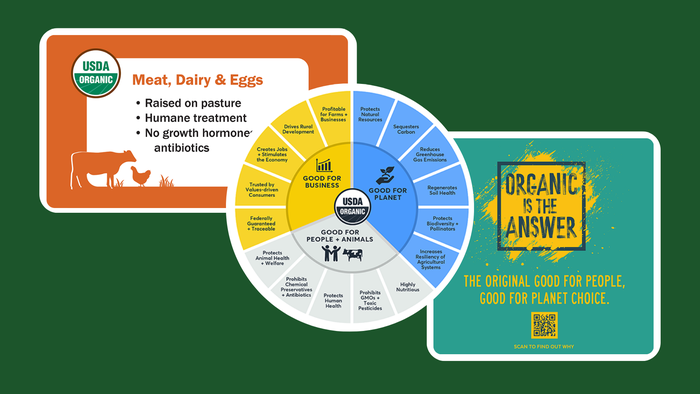
Signs of life emerge across the natural products investment landscapeSigns of life emerge across the natural products investment landscape
As the Federal Reserve ratcheted up interest rates, investment in natural and organic products slowed to a trickle. See when the spigots might open again.
Subscribe and receive the latest updates on trends, data, events and more.
Join 57,000+ members of the natural products community.