Personal Care
Unboxed natural dental products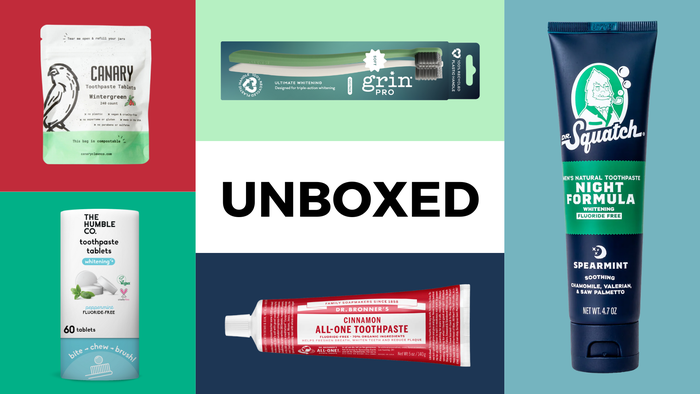
Brands
Unboxed: 12 products for a naturally brighter smileUnboxed: 12 products for a naturally brighter smile
Check out our editors' favorite natural dental products that keep our teeth shiny and healthy.
Subscribe and receive the latest updates on trends, data, events and more.
Join 57,000+ members of the natural products community.