New Hope Network Standards & Guidelines
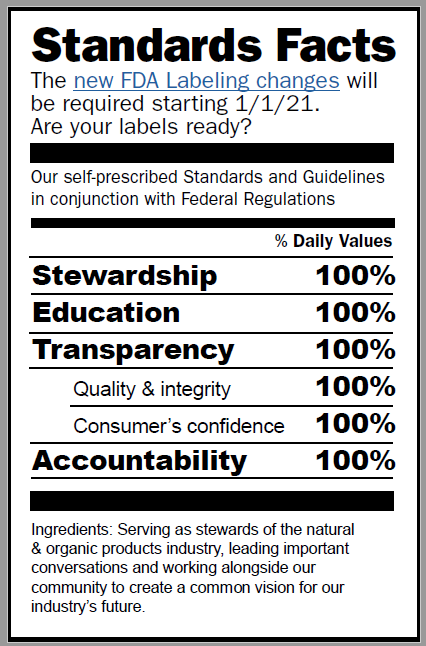
At New Hope Network, we serve as stewards of the natural & organic products industry, leading important conversations and working alongside our community to create a common vision of our industry’s future.
We Believe...

In Stewardship
We have a responsibility to provide thought leadership and to serve our community through constructive, forward-looking dialogue

In Community
We are best served through self-regulation and responsible action, and we must come together to collectively shape the future vision for of our industry and continue striving to serve people and planet

In Transparency
We created our Standards and Guidelines with the goal of serving the best interests of all and evolving as the needs of people and planet evolve, and we focus on education and awareness around key issues when executing our standards and guidelines

Letter from Michelle Zerbib, New Hope Network Standards Director
The New Hope Standards program was initially prompted by the passing of DSHEA, the Dietary Supplement Health & Education Act, in 1994. At that time, the majority of Natural Products Expo exhibits presented dietary supplements and functional ingredients, and the then-leaders of New Hope had the wisdom and foresight to understand how important it would be to support, reinforce and grow industry regulatory compliance. As the rules were being implemented nationwide by the U.S. Food & Drug Administration (FDA), so too was the execution of a new set of Standards for Natural Products Expo exhibitors, standards that would also apply to advertisers, and eventually to the entirety of New Hope Network’s current portfolio.
Through the years, the make-up of exhibitors has shifted and grown to include a wide range of products, particularly foods and beverages. Innovation and manufacturing technologies have also dramatically advanced, driving an ongoing demand for transparency. The Standards program continues to evolve and now encompasses both the applicable federal regulations and our self-prescribed Standards and Guidelines. We continue to strive toward transparency and self-regulation, to maintain a high level of quality and integrity, and to uphold – and increase – the level of consumer confidence in the products promoted through the New Hope Network. Our team looks forward to working with you to further this mission, and we always welcome your thoughts and questions.

Letter from Carlotta Mast, Market Leader
In the natural & organic products industry, we are faced with an increasingly complex landscape of ingredients and technologies, many of which fall outside of the scope of existing federal regulations. At New Hope Network, we are in the unique position to be able to tap into our network of experts and to facilitate the conversation among community members to help us all self-manage to our collective vision of creating a long-term, sustainable and impactful industry.
For many years, we at New Hope Network have operated with a set of guideposts: some come to life as “standards” or requirements for exhibiting in our Natural Product Expos or advertising in our publications. Others are “guidelines” or suggestions that reflect what we believe our retailers and consumers are coming to expect. You can read more about our standards and guidelines here and see the actual policies here.
Going forward, we aspire to lead the conversation on new ingredients and technologies and want to set guideposts that enable our industry to heal and serve people and planet and ensure justice, equity, diversity and inclusion for all. For that, we will want your thoughts, feedback and participation in the dialogue. So please read on, engage and stay in touch. You can always reach us at [email protected].
While the Food and Drug Administration’s has not yet laid out a regulatory pathway for hemp CBD products, New Hope Network is committed to setting industry standards. Learn the most common mistakes on hemp CBD labels. Getting the label correct on a hemp CBD product is tricky, but here’s how to make sure you’re on the up and up.
Market Integrity:
Elevating the integrity of the products and businesses within the markets we serve.
See how fostering consumer trust is of the utmost importance so we can continue to have a successful industry



