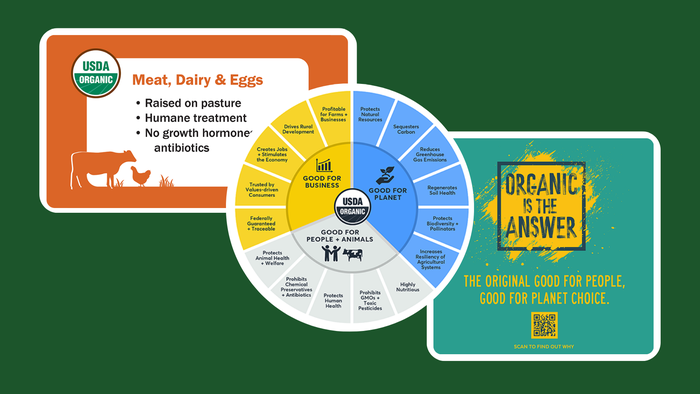
Market Data and Analysis
thumbnail
Market Data and Analysis
Sports-specific products continue to fuel consumers' everyday lifestyleSports-specific products continue to fuel consumers' everyday lifestyle
Powders dominate the sports nutrition and weight management category, which includes bars, pills and beverages. Find out why in this infographic.
Subscribe and receive the latest updates on trends, data, events and more.
Join 57,000+ members of the natural products community.