Market Data and Analysis
Monitor: 'Sports' a low priority for sports nutrition consumers
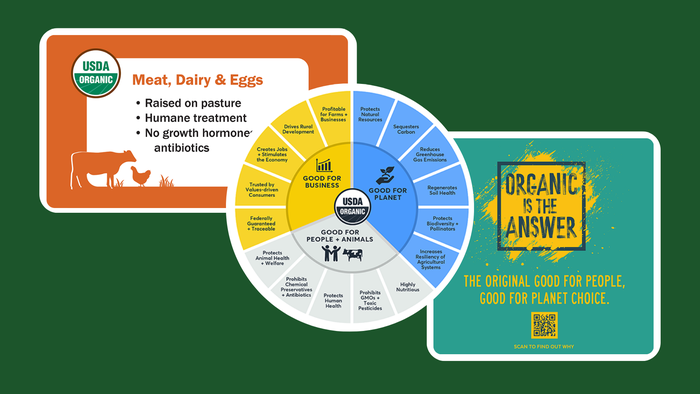
Vitamins and Supplements
Monitor: 'Sports' a low priority for sports nutrition consumersMonitor: 'Sports' a low priority for sports nutrition consumers
NBJ’s new Sports Nutrition and Weight Management Report finds consumers seek sports nutrition for general health more than performance. Find out more.
Subscribe and receive the latest updates on trends, data, events and more.
Join 57,000+ members of the natural products community.