Market Data and Analysis
3 toolkits to help retailers and brands improve organic messaging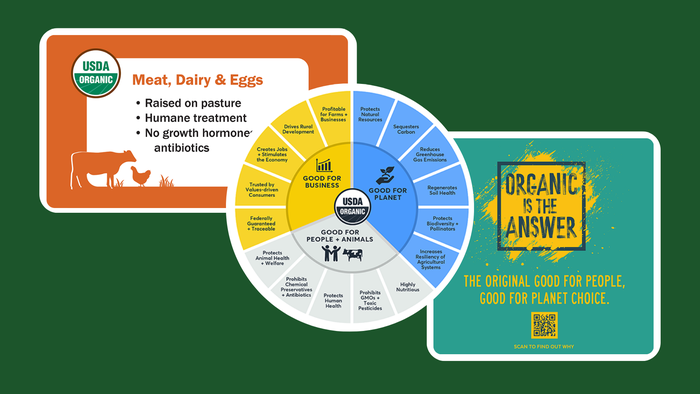
Industry Insights
3 toolkits to help retailers and brands improve organic messaging3 toolkits to help retailers and brands improve organic messaging
As consumers’ values shift, organic messaging must change as well. Learn about three tools retailers and brands can use to educate consumers.
Subscribe and receive the latest updates on trends, data, events and more.
Join 57,000+ members of the natural products community.