
"Back to nature” is what people say when they’re ready to trade in the complications of modern life for a simpler existence. For the FDA, “back to nature” is an order, one that’s anything but simple.
If enforced as stated in the New Dietary Ingredient (NDI) draft guidance released Aug. 11, synthetic botanicals will no longer be considered a food and will be pushed out of the supplement space. The implications are enormous, but the complexities can be brought to focus in the most basic of organisms: algae.
On the leeward side of the Big Island of Hawaii, the near-14,000-foot twin volcanoes of Mauna Loa and Mauna Kea prevent precipitation on the western side of the island. This rain-free zone is ideal for Cyanotech’s open ponds of spirulina and astaxanthin, a promising ingredient for myriad applications. The fairly low-tech system is powered by sunshine, and operates without the use of pesticides and herbicides, creating no soil erosion, fertilizer runoff or water pollution. It is elegant in its simplicity.
Astaxanthin had been growing away in obscurity until Dr. Oz aired a segment in 2012 extolling its health benefits as an antioxidant, anti-inflammatory and vision-improver.
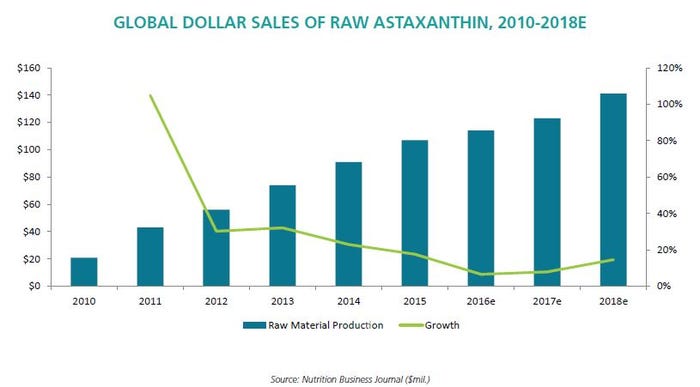
Astaxanthin ingredient suppliers did well with this boom, and thus began the seemingly inevitable competitive wars among one another in a fight for greater market share. Cyanotech even stopped selling its ingredient to manufacturers and went straight to finished product under the BioAstin brand.
But then the seas turned even more turbulent when ingredient giant DSM introduced a “nature-identical” astaxanthin to the market, priced to sell.
Suddenly, the three primary producers of natural astaxanthin—all produced from the Haematococcus pluvialis algal species—put aside their internecine warfare and teamed up in 2014 to form the Natural Algae Astaxanthin Association (NAXA), its mission to educate the public on the benefits of natural astaxanthin, which includes a cocktail of other carotenoids, over the synthetic version being offered from DSM.
“It has become very important for the astaxanthin producers who have developed the market over the last decade to point out how different the various sources are,” said Oran Ayalon, Ph.D., director of R&D at Algatechnologies, a closed-system producer based in Israel. “There are tremendous differences in terms of effectiveness between the different sources.”
The law steps in
Just when you thought astaxanthin was mirroring a typical ingredient war-type situation, with competition both healthy and cuthroat, a new variable has stepped in that could potentially upend the competitive scene.
The FDA’s NDI draft guidance is poised to rock the ingredients world as well as the dietary supplements world in a more seismic way than anything else this side of the passage of DSHEA in 1994—the legislation that initially introduced the concept of NDIs.
More than 20 years later, the FDA has re-issued its interpretation of NDIs (its first issuance was in 2011). In it, the FDA determined that “nature-identical” synthetic botanical molecules—as with DSM’s astaxanthin, but also as with vinpocetine, L-theanine, and some kinds of resveratrol—do not fit the FDA’s definition of a botanical dietary ingredient.
“What we heard in 2011 is the FDA should consider synthetic copies of botanical constituents to be a dietary ingredient,” says Cara Welch, the senior advisor for the office of dietary supplement programs at FDA. “We added one-and-a-half pages of clarification to when synthetic substances of any kind meet the statutory definition of a dietary ingredient, and why. You may not be happy with it but at least you’ll hear a clarification of our position and why that is.”
The FDA determined that DSHEA’s definition of a botanical is, basically, a plant that grows in the ground. Since synthetic versions of these plants do not grow in the ground—even when molecularly identical—they are not plants.
In September, the FDA used that rationale for sending a flurry of letters to producers of vinpocetine, which is used to help with memory functions, but is also produced synthetically at industrial scale, not by pulling periwinkle flowers from the ground. The agency said that vinpocetine does not meet the definition of a dietary ingredient under DSHEA and is excluded from the definition of a dietary supplement under the Food Drug and Cosmetic Act.
Curiously, vinpocetine already enjoys a handful of NDI no-objection letters, which essentially say the FDA has no problem with the safety profile of this ingredient. But in its 2016 guidance, the agency seems determined to walk back this earlier assessment.
This determination against “synthetic botanicals” will significantly shift the ingredients and supplements industry. At a daylong workshop in September produced by the United Natural Products Alliance, based in Salt Lake City, an informal poll of close to 200 individuals in the room that represent a range of supplement manufacturers, more than half of them said they used synthetic botanicals.
“I fully support the NDIs,” said Bob Capelli, executive vice president of global marketing at Algae Health Sciences, a division of BGG, a Chinese company that is growing natural astaxanthin near the Tibetan pleateau. “If you take a chemical formulation of the natural versus synthetic it’s exactly the same, but the molecules are different—the natural is complexed with supportive carotenoids. I’m happy it’s happening.”
As it relates to DSM’s fortunes with synthetic astaxanthin, the company will likely be able to rely on a loophole that should keep its ingredient on the market despite the FDA’s apparent ruling against any synthetic botanical ingredients.
Welch said that synthetic botanicals could “fit under a different provision of a dietary ingredient if this constituent is also in a food supply as a synthetic ingredient.”
One example includes vanillin, the vanilla extract, which has been used for baking. “Vanillin,” said Welch, “whether synthetic or natural, fits the definition of a dietary ingredient.”
Synthetic caffeine is another ingredient that has been in the food supply long enough to qualify.
For synthetic astaxanthin, it has long been used as feed for aquaculture salmon, so it, too, should qualify as an officially recognized dietary ingredient because the farmed fish eat synthetic astaxanthin which is then eaten by humans—ergo synthetic astaxanthin has been in the human food supply.
Of course, Congress could provide a simple fix for these synthetic botanical dilemmas. The FDA’s position could seriously transform the supplements market—under the guise of safety but seemingly instead caught up in the net of regulatory nit-picking definitions. All Congress would need to do is to simply change the definition of dietary ingredient to recognize molecularly identical compounds regardless of their source, even if only via a successful NDI filing with toxicity studies to ensure safety. But the supplements industry is concerned that opening DSHEA to any legislative fixes could open DSHEA to other legislative maneuvers they wouldn’t want.
And so, an open pond filled with red astaxanthin algae becomes an apt metaphor for the future of synthetic botanicals—getting murkier by the moment.
From Nutrition Business Journal's 2016 Supply Chain Issue. For the full issue, including extensive market data and state of the industry analysis, call subscription services at 303-998-9536.
About the Author(s)
You May Also Like





