Consumers: Health products' safety, quality is retailers' burden
Retailers should conduct independent testing to ensure that supplements, personal care products and OTC medications are safe and effective, survey finds.

Manufacturers of dietary supplements and over-the-counter medications are required by law to follow good manufacturing practices, but consumers aren't sure that the law goes far enough, a July survey by NSF International found.
"We’re seeing a trend across categories in the health and wellness sector: Consumers increasingly want retailers to stand behind the products they sell," David Trosin, managing director of Health Sciences Certification at NSF International, said in a press release. "According to this research, consumers not only expect retailers to test products for safety, they want [retailers] to go further and inspect manufacturing facilities for compliance with current good manufacturing practices."
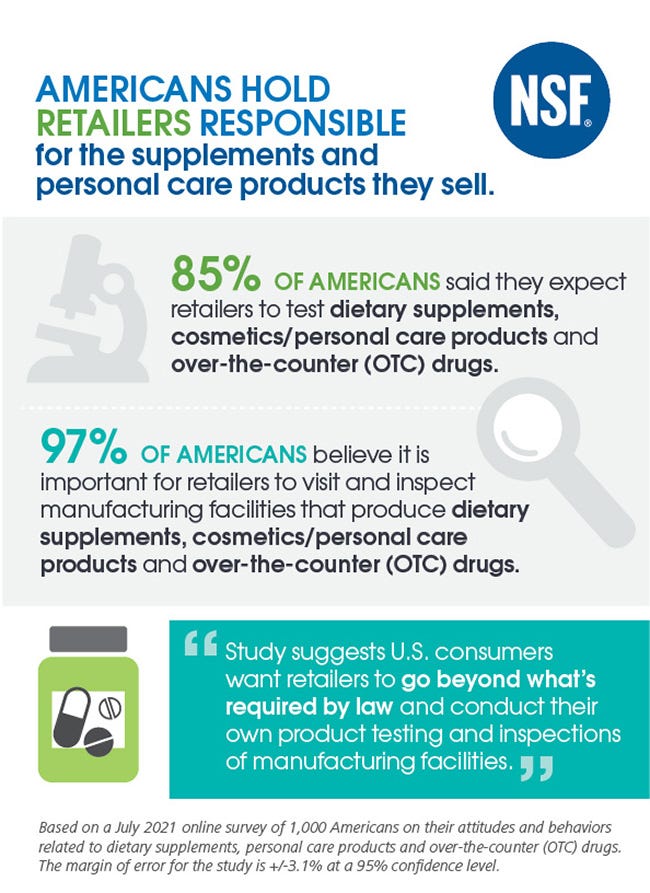
The study finds 97% of Americans said it is important for retailers to visit and inspect manufacturing facilities that produce these products.
In addition, nearly 85% of respondents said they expect retailers to test supplements and other wellness products for safety,
"Failure to comply with GMPs can lead to a host of quality and safety issues. You can get cross contamination of products, mislabeled products, inaccurate formulations and generally unhygienic conditions," Trosin said.
"Everyone in the industry understands the importance of good manufacturing practices and now it’s clear consumers are concerned about this too," he said. But only 32% of respondents said they think retailers actually test supplements and wellness products for safety, according to the survey.
Late last year, Amazon established standards for dietary supplements sold through its e-commerce site. Not everyone in the industry was pleased, however.
Frank Jaksch, co-founder and executive chairman of Chromadex, told NBJ in April that the requirements weren't strict enough.
"Truly ensuring the quality of supplements sold on Amazon would require the implementation of a surveillance or testing program," Jaksch said. "If we use history as our guide, without a mechanism for policing and enforcing these specifications, it essentially does nothing to ensure the quality of products being sold." https://www.newhope.com/regulatory/supplement-industry-responds-new-amazon-requirements
"When retailers develop their own standards that do not align with current manufacturing practices or that limit a company’s ability to work with a specific third-party lab, the result burdens manufacturers and fails to help consumers," Megan Olsen, vice president and general counsel for the Council of Responsible Nutrition, told Nutrition Business Journal in February.
Because differing standards among retailers would complicate manufacturing, the Global Retail and Manufacturer Alliance (GRMA) created a program that incorporates GMP for dietary supplements—a program that provides retailers with a standard by which they can be sure the manufacturers are following the law, Olsen said.
In April, Michael McGuffin, president of American Herbal Products Association, said, "Amazon’s revised dietary supplements compliance program is seen by many supplement brands as moving in a good direction. There is strong support for Amazon taking on an active retailer gatekeeper role and a perception that this will benefit consumers and responsible supplement companies."
McGuffin praised Amazon's new requirement that product labels are visible on each seller’s webpage. "AHPA adopted a similar policy in 2006 to encourage product information be provided prior to purchase, so that consumers know what they are buying," he said.
What do consumers want to know?
When asked, 95% of consumers surveyed said they are concerned about the quality and safety of dietary supplements, personal care products and OTC medications.
What would raise their trust in the products?
62% said independent certification from a health and safety organization.
61% said made in a facility that has been inspected for good manufacturing practices.
44% said lab testing by the retailer.
17% said social media comments and celebrity endorsements.
14% said advertising on TV.
The consumers most likely to demand laboratory testing of supplements, personal care products and OTCs are consumers with children, consumers in urban areas, consumers 23 to 35 years old, and men making over $90,000 per year.
Consumers also were asked what retailers they believed were most likely to sell the highest quality health and wellness products.
62% said national chain stores or pharmacies.
28% said online sellers.
17% said fitness centers.
The online survey of 1,000 representative Americans was conducted in July 2021 on behalf of NSF International. The margin of error for the study is +/-3.1% at a 95% confidence level.
What COVID-19 had to do with this
Not surprisingly, a majority of consumers, 56%, said the COVID-19 pandemic made them more concerned about the safety of supplements, personal care products and OTC medications.
Of course, the pandemic affected supply chains and perhaps opened the door to unscrupulous suppliers getting their products into those supply chains. Most industries have had trouble keeping their supply chains running smoothly. To fill the gaps, they've had to find new suppliers or vendors, NJ Labs CEO Sandra Lee told Whole Foods Magazine in September.
"As a result, opportunistic new companies are now popping up. These companies tend to be inexperienced, and often rush the processes, producing products that may end up containing adulterated materials and ingredients," Lee said. "An independent laboratory with the right quality management system should be unbiased, clear in methodology and traceable. Laboratories that have a foundation based on quality are better positioned in identifying and addressing issues with compromised supplies."
About the Author(s)
You May Also Like




