Radicle predictions: 5 trends in healthtech, dietary supplements
The co-founders of Radicle Science lay out their expectations for the supplement industry in 2024. Dive in to their forecasts.

From enforcement of the new FTC guidance, the Dietary Supplements Access Act, supply chains and pricing challenges, and authenticity issues plaguing online retailers, 2023 was a roller coaster ride for the supplement industry. Our prediction is that 2024 will not disappoint when it comes to new industry trends, market disruptions, and of course, plenty of controversy. Here’s our take on the top five predictions for 2024 with game-changing potential:
The rise of femtech: A new era in women's health and nutrition
In 1977, an FDA policy recommended excluding women of childbearing potential from clinical drug trials. Not until 1993 did the FDA explicitly reverse this exclusion. Since then, there has been an increase in research on women and extensive growth in women’s health products.
Globally, about 37% of the femtech market belongs to pregnancy, nursing and reproductive health and contraception subsectors. While products supporting pregnancy and fertility are essential, the real growth story is the potential of everyday dietary supplements being formulated and clinically proven specifically for uniquely female needs like menstrual or menopausal symptoms or women’s sleep or mood issues. Moreover, the historically neglected category of women’s sports nutrition is now growing faster than ever. For 2024, expect this under-represented, under-studied, and under-supplemented population to finally have its day of reckoning as it gets the quality and efficacy of dietary supplements that it rightfully requires and deserves.
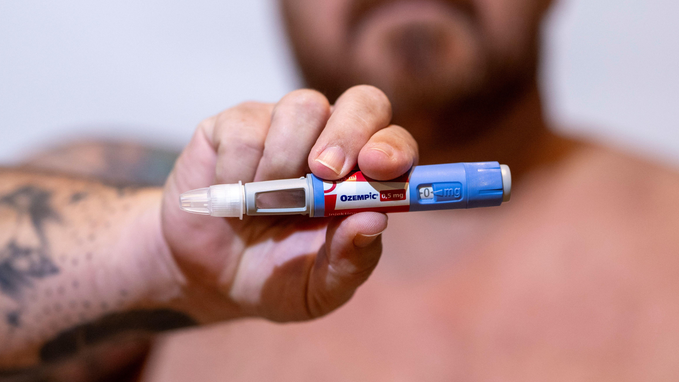
The Ozempic effect: Demand for off-label use of pharmaceutical products
We are beginning to see retail implications from the Ozempic weight loss frenzy and the resulting changes in consumer shopping trends. What hasn’t been explored is the potential threat to the supplement industry resulting from the societal acceptance, desire, and demand for the off-label use of pharmaceutical products for health conditions, such as weight loss, where consumers historically turned to supplements.
Another threat is the demand for secondary access points for similar versions of these drugs: compounding laboratories. While compounding laboratories are regulated by the FDA, compounded drugs are not FDA-approved per se. This means the FDA does not verify the safety or effectiveness of compounded drugs. Are consumers aware of these risks? Given the growth of compounding labs offering weight-loss medication alternatives and resulting lawsuits, it doesn't seem like it. Why are consumers paying hundreds, sometimes thousands of dollars out of pocket each month for these solutions? Because they are perceived to be proven effective with a clinical trial.
Competition for health areas historically addressed by dietary supplements? Check. Effectiveness? Check. Underenforced space? Check. Grab your popcorn! Let’s see how this unfolds in 2024. Better yet, read the writing on the wall, get in the driver’s seat, head it off at the pass and secure a trial for your dietary supplement to elevate consumer trust with clinical proof.
Advancing health literacy: Next-gen tools to enhance supplement adherence
About 92% of supplement users believe dietary supplements are crucial for health, according to an Ipsos survey for the Council for Responsible Nutrition that was released in October. Yet poor adherence to product usage recommendations remains an issue, negatively impacting consumer health benefits and repeat purchases. The World Health Organization reported in 2003 that medication adherence is a worldwide problem, with only 50% adherence to chronic medications. Adherence to antibiotic therapies is a little better, with 60.8% of survey participants “always adhering to antibiotics recommendations.” Dietary supplements face bigger challenges when it comes to adherence, a National Institute of Health study reported in 2022. In 2024, mobile apps and gamification are poised to enable adherence reminders and allow consumers to easily track their personal health journeys, arming them with real-world results boosting trust, repeat purchase, and lifetime value, benefiting both consumer health literacy and brand profits—a true win-win.
AI: Adapting to your new co-pilot
We are all just beginning to get a glimpse into all the ways that artificial intelligence can be a valuable co-pilot in the workplace and at home. The dietary supplement industry is no exception, contributing to both excitement and fear! Studies show that challenges with AI aren't due to its current capabilities, but rather due to the workforce lacking understanding or feeling threatened. A UKG study of 4,000 employees and executives across 10 countries found that 54% don't know how their companies use AI. The concern about jobs being threatened is more perception than reality for now, as AI is used more for automating tasks than replacing entire jobs, though change is coming. Executives estimate 70% of their workforce will use AI for task automation or augmentation by 2028. So, embrace AI and adapt; it's here to stay. As X Prize founder Peter Diamandis said, “There will be two kinds of companies at the end of this decade: Those who are fully utilizing AI, and those who are out of business.”
Expanding FDA oversight: What’s next after the Amazon debacle?
There is no question that Amazon was the poster pin-up for the FDA’s warning letters in 2023. The most recent, from Dec. 20, cited the online retailer for “undeclared and potentially harmful active pharmaceutical ingredients,” and that Amazon is “responsible for introducing or delivering for introduction into interstate commerce products that are unapproved new drugs.” It concludes:
You are responsible for preventing their recurrence or the occurrence of other violations. It is your responsibility to ensure that your firm complies with all requirements of federal law, including FDA regulations. Failure to adequately address this matter may result in legal action including, without limitation, seizure and/or injunction.
However, when it comes to compliance, the issue of unsubstantiated claims, undeclared ingredients and introduction of non-compliant products into interstate commerce isn’t exclusively an Amazon issue. Many of these products are carried by other brick and mortar grocery stores, online-retailers and local co-ops. Because of repeat FDA violations, expect a crackdown in 2024. This could include making Amazon an example and increasing the severity of the violation repercussions or extending the precedent to include other online or brick and mortar retailers to clarify FDA’s compliance stance once and for all. Compliant brands, take notice and heed the call. When these non-compliant products inevitably get pulled from shelves, this creates more shelf-space opportunities for legitimate brands.
If that’s not enough, 2024 also brings us the spectacle that is a pivotal election year. The dynamics of dietary supplements and politics will become even more intriguing depending on who controls the Congress and, ultimately, the presidency. That said, buckle up, we’re in for an interesting ride.
Radicle Science is an AI-powered healthtech company, enabling dietary supplements to clinically prove their true effects beyond placebo at unprecedented affordability, speed and scale with their transformative Proof-as-a-Service approach. Learn more at www.radiclescience.com.
Have some big ideas or thoughts to share related to the natural products industry? We’d love to hear and publish your opinions in the newhope.com IdeaXchange. Check out our submission guidelines.
Read more about:
IdeaXchangeAbout the Author(s)
You May Also Like






