Regulatory
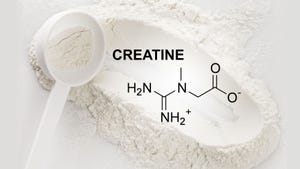
Amazon mandates testing for sex, sports and weight-management products
Retailers
Amazon mandates testing for sex, sports and weight-management productsAmazon mandates testing for sex, sports and weight-management products
Supplements in three categories must be verified through third-party testing to be sold on Amazon going forward. Get the details of this new policy.
Subscribe and receive the latest updates on trends, data, events and more.
Join 57,000+ members of the natural products community.